Presenter Name: Dallin Billings
Description
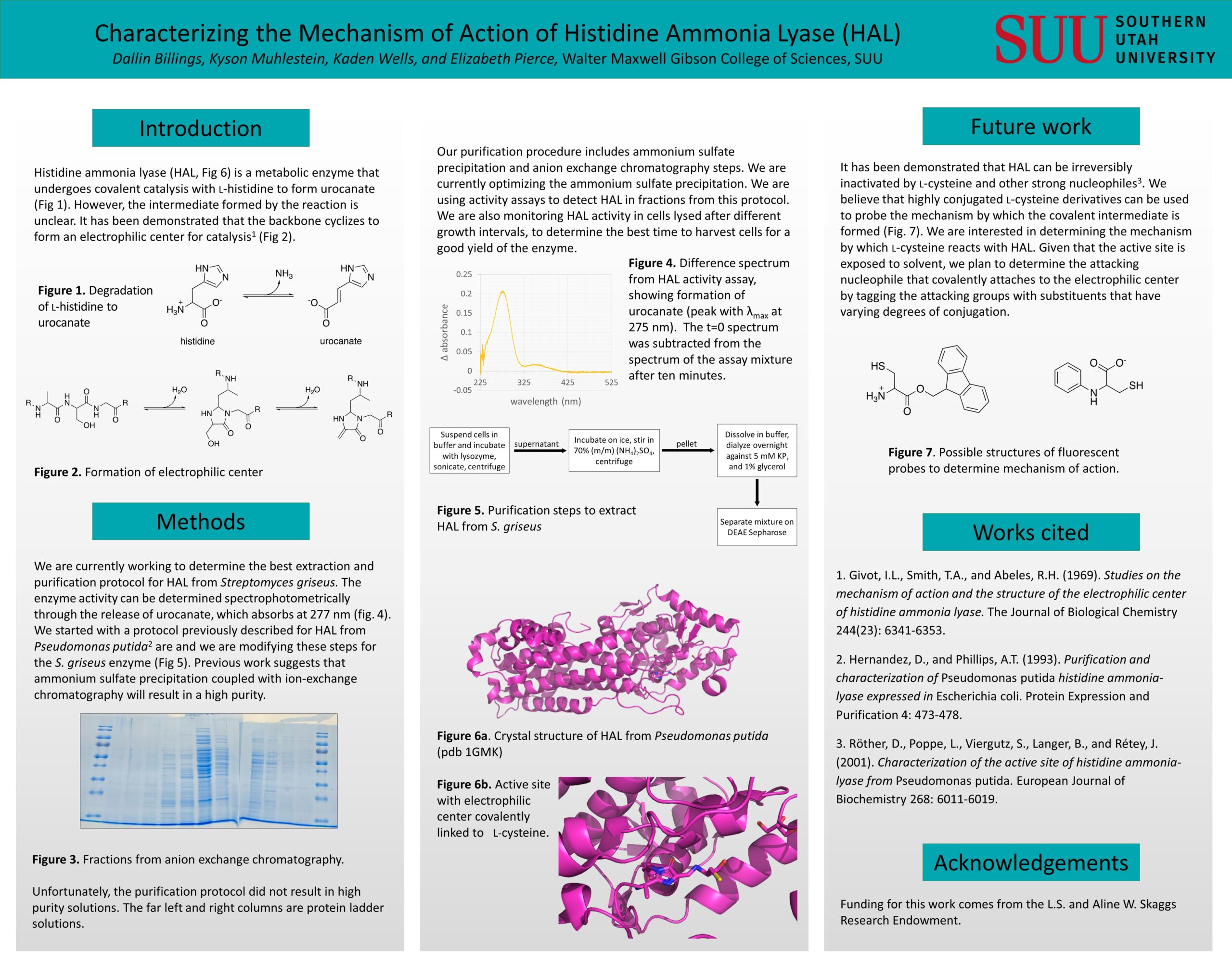
Histidine ammonia lyase (HAL) is a metabolic enzyme whose mechanism has not been fully characterized. Deamination requires the presence of an electrophilic prosthetic group (MIO), which is formed by cyclization of the protein backbone involving alanine, serine, and glycine. This is similar to the cyclization reaction that forms the chromophore in green fluorescent protein (GFP), though the process in HAL positions the active site exposed to the solvent, whereas the GFP chromophore is buried in the protein. Backbone cyclization results in the formation of an alkene by elimination of the serine hydroxy group. This serves as the electrophile for the enzymatic mechanism. The covalent enzyme-substrate intermediate during the catalysis of L-histidine to ammonia and urocanate is unclear, however, there are two main hypotheses of the intermediate's structure-namely an N-MIO complex formed from nucleophile attack of the amino group in the substrate, and a Friedel-Crafts complex formed by nucleophile attack from the aromatic ring of the substrate. We are optimizing the purification of HAL from Streptomyces griseus using ammonium sulfate precipitation and ion exchange and hydrophobic interaction chromatography.
Once protein purification methods have been refined, we plan to probe the protein using analogs of L-cysteine (shown to irreversibly inactivate the enzyme) and to monitor changes to the enzyme activity and UV-visible spectrum. One preliminary focus of interest is to determine whether a nitrogen or sulfur serves as the attacking nucleophile in the primary step of the inhibition mechanism, which might be observed spectroscopically with L-cysteine derivatives using highly conjugated substituents near the sulfur and nitrogen heteroatoms. Once we have determined the attacking nucleophile, we would like to see if this correlates to the mechanistic nucleophile in the catalysis on histidine. Additionally, we would like to determine if HAL can be utilized to form fluorescent chromophores after the addition of L-cysteine derivatives with highly conjugated systems.
Once protein purification methods have been refined, we plan to probe the protein using analogs of L-cysteine (shown to irreversibly inactivate the enzyme) and to monitor changes to the enzyme activity and UV-visible spectrum. One preliminary focus of interest is to determine whether a nitrogen or sulfur serves as the attacking nucleophile in the primary step of the inhibition mechanism, which might be observed spectroscopically with L-cysteine derivatives using highly conjugated substituents near the sulfur and nitrogen heteroatoms. Once we have determined the attacking nucleophile, we would like to see if this correlates to the mechanistic nucleophile in the catalysis on histidine. Additionally, we would like to determine if HAL can be utilized to form fluorescent chromophores after the addition of L-cysteine derivatives with highly conjugated systems.
University / Institution: Southern Utah University
Type: Poster
Format: In Person
Presentation #B57
SESSION B (10:45AM-12:15PM)
Area of Research: Science & Technology
Email: dallin.billings@gmail.com
Faculty Mentor: Elizabeth Pierce